Counting of red blood cells (RBC)
Apparatus and reagents
- Syringe
- Lancet
- Alcohol
- Cotton
- Hayem's fluid/normal saline solution
- Coverslip
- Microscope
- Hemocytometer
Hemocytometer
It contains a counting chamber, RBC diluting pipette, and WBC diluting pipetteHemocytometer counting chamber principle
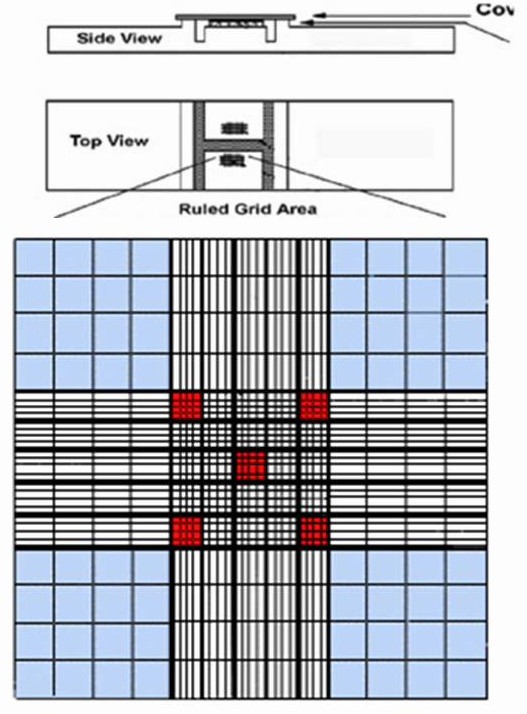
- Each of the two counting areas in a hemocytometer counting chamber is 9 mm2 and is divided into nine squares.
- The four corner squares are used for counting leucocytes and are divided into 16 smaller squares to make the counting process easier.
- The center 1 mm2 is divided into 16 small squares (1/25 mm2) and each of these is further subdivided into 16 smaller squares.
- The 1/25 mm2 squares are bounded by double lines and are used for counting red blood cells.
RBC Pipette
- It has a narrow glass stem, graduated in tenths, with three markings 0.5, 1.0 below the bulb, and 101 above the bulb.
- The glass capillary tube widens into a small bulb containing a red glass bead.
- The bulb narrows again into a glass capillary tube and at this point, it is marked 101.
- A thin rubber tube is attached beyond this with a plastic mouthpiece.
- The red bead helps in mixing the contents of the bulb and for quick identification of the RBC pipette.
- The marking 0.5, 1.0, and 101 indicate the volume of blood and diluent.
Procedure
- Place the hemocytometer on the microscope stage and examine it at low power (4X) objective to identify the counting areas.
- Use some higher power (10X) objective to find the center 1 mm2.
- Adjust a higher power (40X) objective to focus on smaller 1/25 mm2.
- Place a few millimeters a Hayem's solution in a clean glass beaker.
- Attach a rubber suction tube to the upper end of the RBC pipette and place the mouthpiece b/w your lips.
- Before sucking the blood, mix the sample gently so that RBCs are uniformly distributed in the plasma and are not settled at the bottom.
- Keeping the pipette in a horizontal position, insert the tip into the blood.
- Suck gently on rubber tube to draw blood up to exactly 0.5 marks on the pipette.
- Wipe the excess blood from the pipette tip with a tissue or cotton by touching the pipette tip to the tissue to draw the blood back to the 0.5 marks.
- Place the pipette tip in the diluting fluid and draw the fluid to exactly the 101 marks.
- Remove the rubber tubing, cover the two ends of the pipette with your thumb and forefinger.
- Move the pipette in a circular figure-eight motion for 2 minutes.
- After diluting the cells and keeping it for 5 minutes, blow out about 3 to 5 drops to remove the diluting fluid from the stem of the pipette.
- Place a coverslip over the counting area of the hemocytometer.
- Touch the tip of the pipette to the junction of the cover-slip and the hemocytometer.
- Allow 2 minutes for the cells to settle before beginning the count.
- Focus the center square of the counting area using high power (40X).
- Count the number of red cells in five (4 corners and 1 middle square each with 16 squares i.e., total 80 smallest squares).
Calculation
Calculate the number of RBCs per cubic milliliters of blood by taking into account the following multiplication factor.Multiplication factor
- The blood was diluted 200 times in the pipette, therefore, multiply the average number pr square by 200.
- The depth of the counting chamber is 0.1 mm, therefore, multiply by 10.
- The square counted was only 1/25 the area of the center square., therefore, multiply by 25.
No. of RBCs. = RBCs counted X 50,000

Comments
Post a Comment